Background: Allogeneic hematopoietic cell transplantation (alloHCT) remains the only curable treatment option for several hematological malignancies. Preparative and graft-versus-host disease (GVHD) prophylactic regimens combined with graft-versus-leukemia effect (GVL) are important in preventing graft rejection, disease relapse and GVHD; and subsequently impact the success of this life saving procedure. Choice of regimen depends on patient, donor/graft source and disease characteristics.
Objectives: Assess the outcomes of patients who underwent haploidentical (haplo) or mismatched unrelated donor (MMUD) HCT with myeloablative conditioning (MAC) regimen using radiation in combination with fludarabine (FLU) with PTCy as higher intensity GVHD prophylaxis and evaluate the feasibility of a fludarabine and fractionated total body irradiation (FLU/FTBI)-based regimen in both donors.
Study Design: Retrospective analysis to study the outcomes of patients receiving FLU/FTBI as myeloablative conditioning for mismatched (related or unrelated) donor HCT combined with post-transplant cyclophosphamide (PTCy) as GVHD prophylaxis.
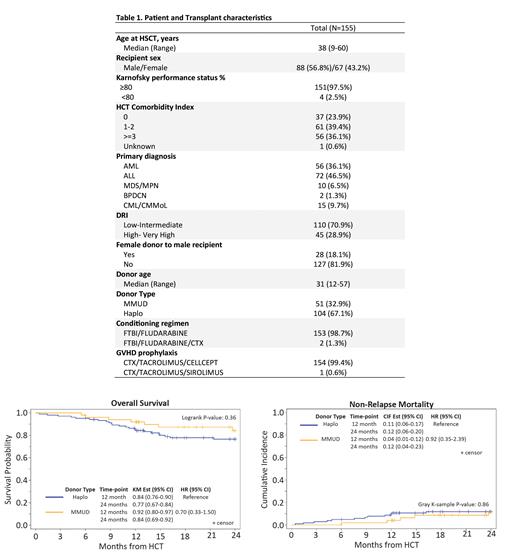
Results: One hundred and fifty-five consecutive patients undergoing HCT at City of Hope (COH) from January 2015 to December 2021 were included. Median age was 38 years (range 9-60) and 56.8% of patients were male and 97.5% had a Karnofsky performance status (KPS) ≥ 80. HCT comorbidity index was ≥ 3 in 36.1% cases and 29% with a disease risk index (DRI) of high/very high. Disease diagnosis included acute lymphoblastic leukemia (ALL) (46.5% of cases) and acute myeloid leukemia (AML) (36.1% of cases). The median donor age was 31-years with 67.1% haplo donors and 32.9% mismatched unrelated donors (MMUD). MMUD HCT led to faster neutrophil recovery (15 vs 16 days, p= 0.01) and platelet count recovery (18 vs 23 days, p= 0.029). Day 100 cumulative incidence of grade II-IV and III-IV acute GVHD had no statistical difference between the two cohorts. With a median follow-up of 24 months (range 3 to 81), 100-day and 1-year NRM was 1.9% and 8.5%, respectively. There was no difference in the NRM between MMUD and haplo (HR 0.92, CI (0.35,2.39), p=0.86). Two-year relapse rate and DFS were 11.8% and 76.9%, respectively. In this study, 7 out of 51 patients in the MMUD group and 17 out of 104 patients in the haplo group relapsed with no statistically significant difference between the groups (HR 0.82, CI: (0.35,1.97), p=0.67). At a median follow-up of 2 years, GRFS was 60% (95% CI 0.51- 0.67). There was no statistically significant difference in GRFS between the MMUD and haplo groups (HR 0.76, CI (0.44,1.32), p=0.33). Two-year OS was 80.1%. There were no DFS or OS differences between the MMUD and haplo groups. On multivariate analysis, age less than 40 and low to intermediate DRI showed a DFS benefit (p = 0.004 and 0.029 respectively). None of the demographic factors affected OS or GRFS. Disease risk index was the only factor on multivariate analysis affecting relapse rates with higher risk of relapse with higher DRI (p= 0.017) and extensive chronic GVHD risk (p= 0.014) with no impact on acute GVHD.
Conclusions: FLU/FTBI followed by PTCy for GVHD prophylaxis is a well-tolerated myeloablative regimen in mismatched donors with acute leukemia or myelodysplastic syndrome (MDS) with low transplant related morbidity and mortality and has produced promising HCT outcomes.
Disclosures
Koller:treadwell therapuetics: Consultancy, Other: safety review committee; takeda: Consultancy, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sandhu:Autolus Therapeutics: Consultancy; City of Hope Medical Center: Current Employment. Aldoss:Takeda: Consultancy; Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Jazz: Consultancy; Sobi: Consultancy; KiTE: Consultancy. Ali:Karyopharm: Consultancy; GSK: Consultancy; Pharmaessentia: Consultancy; Blueprints: Speakers Bureau; BMS: Speakers Bureau; Incyte: Research Funding. Salhotra:BMS: Research Funding; OrcaBio: Research Funding; Jazz Pharma: Research Funding; Rigel Pharma: Research Funding; Sanofi: Speakers Bureau; Sobi: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Kura Oncology: Research Funding. Aribi:Kite, a Gilead Company: Consultancy; Seagen: Consultancy. Artz:Abbvie: Consultancy; Magenta Therapeutics: Other: Advisory Board; Astra Zeneca: Other: Advisory Board; Radiology Partner: Current equity holder in private company, Other: Spouse equity interest. Becker:Accordant Health Services: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Research Funding; Pfizer: Research Funding; GPCR Therapeutics: Research Funding. Pullarkat:Amgen: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Stein:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding. Nakamura:NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees; Napajen: Consultancy; International Consortium: Other: consortium chair; Mt. Sinai: Other: Acute GVHD; Blue Bird: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: research collaboration; Miyarisan: Research Funding; NCCN: Other: guideline panel for HCT; Leukemia & Lymphoma Society: Other: grant reviewer; BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; Omeros: Consultancy; Sanofi: Consultancy. Al Malki:Tscan: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal